Characterization of a Pseudorabies virus mutant lacking the pUS3 kinase and the tegument protein pUL21 in vitro and in vivo and the evaluation of an improved animal model for human Herpes Simplex Encephalitis
Seiten
2020
VVB Laufersweiler Verlag
978-3-8359-6881-3 (ISBN)
VVB Laufersweiler Verlag
978-3-8359-6881-3 (ISBN)
- Keine Verlagsinformationen verfügbar
- Artikel merken
Alphaherpesviruses are neuroinvasive viruses with the ability to cause severe diseases in humans and animals. HSV-1 is a human pathogen known to sporadically induce fronto-temporal meningoencephalitis, which is associated with high mortality rates and long lasting sequelae such as chronic or relapsing inflammation, cognitive alterations or epilepsy. PrV is an animal pathogen causing CNS disorders primarily in piglets. In non-porcine species PrV infection leads to severe itching followed by automutilation and death of the infected and diseased animal. After primary replication in epithelial cells, HSV-1 and PrV infect sensory neurons in which they are transported to peripheral ganglia and towards the CNS. In the past, the function of different PrV proteins for neuroinvasion has been studied in mice using corresponding viral gene deletion mutants. These studies revealed that infection with a PrV mutant lacking the tegument protein pUL21 and the pUS3 kinase led not only to a significantly prolonged survival time but unlike to infection with PrV wildtype or other mutants, which was always fatal, most mice survived the infection.
In this thesis, this phenotype was studied in detail. To test whether the effect resulted from the absence of the pUS3 kinase function of or from the absence as virion structural component, virus mutants PrV-US3Δkin and PrV-ΔUL21/US3Δkin were constructed and compared to PrV-ΔUS3 and PrV-ΔUL21/US3. In addition to the influence of the kinase-active pUS3, the function of the two described isoforms, pUS3-S and pUS3-L, was analyzed. In vitro data showed that while pUS3-L plays no major role in the viral replication cycle, the abundant, catalytically active short isoform of pUS3 is required for efficient nuclear egress and production of infectious progeny. However, the accumulations of primary virions in huge herniations of the INM described for mutants lacking US3 are not the cause for the observed drop in virus titers. PrV-ΔUS3, lacking the open reading frame completely, or PrV-US3Δkin, carrying only a single nucleotide exchange resulting in inactivation of the kinase activity, showed comparable phenotypes not only in cell culture but also in the mouse indicating that the enzymatic function but not pUS3 as structural virion component are important. Corresponding phenotypes in cell culture and for survival times in mice were also evident for the mutants lacking in addition UL21 (PrV-ΔUL21/ΔUS3, PrV-ΔUL21/US3Δkin).
Based on these data, PrV-ΔUL21/US3Δkin was used to analyze the pathobiological mechanism in comparison to PrV-Kaplan and the corresponding single mutants (PrV-US3Δkin, PrV-ΔUL21) in mice. As reported before for PrV-ΔUL21/US3 (Maresch 2011), and in contrast to mice infected with wildtype PrV or the single mutants, nearly all PrV-ΔUL21/US3Δkin infected animals survived.
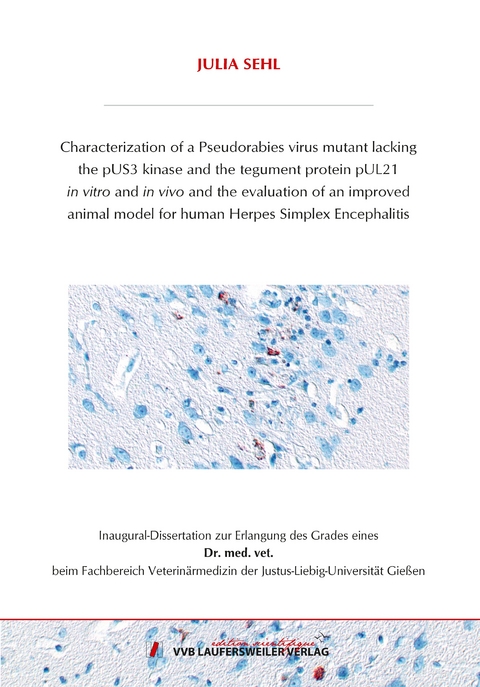
In an extended experiment, mice infected with PrV-ΔUL21/US3Δkin and the control viruses were euthanized and thoroughly studied at different time points after infection. Viral spread and inflammatory response were assessed histologically and correlated to the clinical status. Besides a slight delay in the onset of clinical signs and neuroinvasion in PrV-US3Δkin infected mice, no difference to mice infected with PrV-Kaplan was evident indicating that the pUS3 kinase obviously has no role in neuroinvasion or pathogenicity. PrV-Kaplan and PrV-US3∆kin showed fast neuronal spread and occasionally induced only very mild inflammatory reaction, which might be due to the short time between infection and death of the animals. Infection with PrV-ΔUL21 resulted in delayed, but more widespread neuroinvasion and pronounced inflammation in the ganglia and mild inflammatory response in the brainstem, which correlates with the extended survival time. However, all mice infected with PrV-ΔUL21 developed severe clinical signs and had to be euthanized. PrV-ΔUL21/US3Δkin spread even slower than PrV-ΔUL21, but invaded the cerebral cortex, mainly the frontal and temporal lobe starting 8 d p.i. A fulminant lymphohistiocytic reaction developed, which was first detected in the ganglia and brainstem, but was more pronounced in the telencephalon affecting the temporal and frontal lobe starting 9 d p.i. With increasing inflammation, also behavioral abnormalities occurred in mice, which culminated between 9 to 14 d p.i., whereas mild pruritus and skin lesions developed after 6 d p.i. While viral antigen was no longer detectable in mice sacrificed later than 15 d p.i., meningoencephalitis was present in mice examined at the end of the experiment at day 21 p.i. Despite widespread neuroinvasion and concomitant meningoencephalitis mice were able to survive an infection with PrV ΔUL21/US3Δkin, which had not been reported for any other PrV mutant.
Although the mode of interplay between pUL21 and the kinase activity of pUS3 remained enigmatic, the most important result of this study is that PrV-ΔUL21/US3Δkin infected mice showed unexpected but striking analogies to human HSE including fronto-temporal, lesion-associated virus tropism combined with behavioral abnormalities, which in combination could not be achieved in other animal models. Furthermore, skin lesions and the presence of myelitis in PrV-ΔUL21/US3Δkin infected mice partially resembled the disease caused by VZV in humans.
With PrV-ΔUL21/US3Δkin infection in mice as model, detailed investigations on herpesvirus neuroinvasion, neuronal spread, and immune reaction in the brain but also advanced neuronal tracing is possible that will significantly contribute to our understanding not only of herpesvirus pathobiology but also of neuroanatomical networks.
In this thesis, this phenotype was studied in detail. To test whether the effect resulted from the absence of the pUS3 kinase function of or from the absence as virion structural component, virus mutants PrV-US3Δkin and PrV-ΔUL21/US3Δkin were constructed and compared to PrV-ΔUS3 and PrV-ΔUL21/US3. In addition to the influence of the kinase-active pUS3, the function of the two described isoforms, pUS3-S and pUS3-L, was analyzed. In vitro data showed that while pUS3-L plays no major role in the viral replication cycle, the abundant, catalytically active short isoform of pUS3 is required for efficient nuclear egress and production of infectious progeny. However, the accumulations of primary virions in huge herniations of the INM described for mutants lacking US3 are not the cause for the observed drop in virus titers. PrV-ΔUS3, lacking the open reading frame completely, or PrV-US3Δkin, carrying only a single nucleotide exchange resulting in inactivation of the kinase activity, showed comparable phenotypes not only in cell culture but also in the mouse indicating that the enzymatic function but not pUS3 as structural virion component are important. Corresponding phenotypes in cell culture and for survival times in mice were also evident for the mutants lacking in addition UL21 (PrV-ΔUL21/ΔUS3, PrV-ΔUL21/US3Δkin).
Based on these data, PrV-ΔUL21/US3Δkin was used to analyze the pathobiological mechanism in comparison to PrV-Kaplan and the corresponding single mutants (PrV-US3Δkin, PrV-ΔUL21) in mice. As reported before for PrV-ΔUL21/US3 (Maresch 2011), and in contrast to mice infected with wildtype PrV or the single mutants, nearly all PrV-ΔUL21/US3Δkin infected animals survived.
In an extended experiment, mice infected with PrV-ΔUL21/US3Δkin and the control viruses were euthanized and thoroughly studied at different time points after infection. Viral spread and inflammatory response were assessed histologically and correlated to the clinical status. Besides a slight delay in the onset of clinical signs and neuroinvasion in PrV-US3Δkin infected mice, no difference to mice infected with PrV-Kaplan was evident indicating that the pUS3 kinase obviously has no role in neuroinvasion or pathogenicity. PrV-Kaplan and PrV-US3∆kin showed fast neuronal spread and occasionally induced only very mild inflammatory reaction, which might be due to the short time between infection and death of the animals. Infection with PrV-ΔUL21 resulted in delayed, but more widespread neuroinvasion and pronounced inflammation in the ganglia and mild inflammatory response in the brainstem, which correlates with the extended survival time. However, all mice infected with PrV-ΔUL21 developed severe clinical signs and had to be euthanized. PrV-ΔUL21/US3Δkin spread even slower than PrV-ΔUL21, but invaded the cerebral cortex, mainly the frontal and temporal lobe starting 8 d p.i. A fulminant lymphohistiocytic reaction developed, which was first detected in the ganglia and brainstem, but was more pronounced in the telencephalon affecting the temporal and frontal lobe starting 9 d p.i. With increasing inflammation, also behavioral abnormalities occurred in mice, which culminated between 9 to 14 d p.i., whereas mild pruritus and skin lesions developed after 6 d p.i. While viral antigen was no longer detectable in mice sacrificed later than 15 d p.i., meningoencephalitis was present in mice examined at the end of the experiment at day 21 p.i. Despite widespread neuroinvasion and concomitant meningoencephalitis mice were able to survive an infection with PrV ΔUL21/US3Δkin, which had not been reported for any other PrV mutant.
Although the mode of interplay between pUL21 and the kinase activity of pUS3 remained enigmatic, the most important result of this study is that PrV-ΔUL21/US3Δkin infected mice showed unexpected but striking analogies to human HSE including fronto-temporal, lesion-associated virus tropism combined with behavioral abnormalities, which in combination could not be achieved in other animal models. Furthermore, skin lesions and the presence of myelitis in PrV-ΔUL21/US3Δkin infected mice partially resembled the disease caused by VZV in humans.
With PrV-ΔUL21/US3Δkin infection in mice as model, detailed investigations on herpesvirus neuroinvasion, neuronal spread, and immune reaction in the brain but also advanced neuronal tracing is possible that will significantly contribute to our understanding not only of herpesvirus pathobiology but also of neuroanatomical networks.
| Erscheinungsdatum | 22.09.2020 |
|---|---|
| Reihe/Serie | Edition Scientifique |
| Sprache | englisch |
| Maße | 146 x 210 mm |
| Gewicht | 134 g |
| Themenwelt | Veterinärmedizin |
| Schlagworte | Doktorarbeit • Uni • Wissenschaft |
| ISBN-10 | 3-8359-6881-5 / 3835968815 |
| ISBN-13 | 978-3-8359-6881-3 / 9783835968813 |
| Zustand | Neuware |
| Informationen gemäß Produktsicherheitsverordnung (GPSR) | |
| Haben Sie eine Frage zum Produkt? |
Mehr entdecken
aus dem Bereich
aus dem Bereich
A Practical Guide
Buch | Hardcover (2024)
Wiley-Blackwell (Verlag)
CHF 204,30
Buch | Hardcover (2024)
Wiley-Blackwell (Verlag)
CHF 174,35
Buch | Softcover (2024)
British Small Animal Veterinary Association (Verlag)
CHF 119,95